This is a summary of the course Introduction to Biology - The Secret of Life.
Molecular composition of cells
Atomic composition of life is pretty similar across all living organisms. At the level of atomic composition you are
- 63% Hydrogen (H)
- 24% Oxygen (O)
- 10% Carbon (C)
- 1.4% Nitrogen (N)
- 0.2% Phosphorus (P)
- <0.1% Sulfur (S)
- Trace amounts of ions - Calcium, Chloride, Potassium, Sodium, Magnesium, Manganese, Selenius
Covalent Bonds
Covalent bonds are shared pairs electrons between two different atoms. There are different kinds of covalent bonds determined by how many pairs of electrons are shared between two atoms.
- Single Bonds
- Double Bonds
- Triple Bonds
The strength of a bond is measured by how much energy it takes to break the bond (measured in $kcal/mol$).
Covalent bonds require around $80 \cdot kcal/mol$ to break.
The maximum number of covalent bonds each atom can engage in is:
| Atom | Maximum Bonds |
|---|---|
| Hydrogen | 1 |
| Carbon | 4 |
| Oxygen | 2 |
| Nitrogen | 3 (sometimes 4) |
| Phosphorus | 5 |
| Sulfur | 6 |
- Sharing may not be equal - some nuclei of some atoms pull harder. Bonds in which electrons are shared equally are called non-polar bonds and bonds in which electron are not shared equally are called polar bonds.
- The bond is probabilisticly moving around, but not necessarily equally shared. The measure of how much an atom “pulls” on an electron is called electronegativity. Oxygen and Nitrogen are much more electronegative than Carbon and Hydrogen. We may write: $$O,N \gg C,H$$
- Covalent bonds don’t break up at random.
Non-Covalent Bonds
Hydrogen Bonds
Molecules may be polar if they are formed by polar bonds. Molecules are like little magnets which some sides which a probabilisticly more / less charged. The negative sides of molecules can be attracted to the positive sides of other molecules. These form bonds which we call hydrogen bonds.
These bonds are formed when a polar hydrogen in one functional group is near an electronegative atom (like N or O) in another functional group.
These bonds are weaker than covalent bonds and require around $5 kcal/mol$ to break.
Ionic Bonds
Ionic bonds are formed when electrons are completely transferred from one atom to another. It is usually formed between two oppositely charged ions (atoms with a net charge) or between two atoms of sharply different electronegativites.
Van der Waals bonds
Van der Waals bonds are caused by correlations in the fluctuating polarizations of nearby particles.
Hydrophobic forces
Imagine a glass of water which contains a lot of $H_2 O$ molecules which create hydrogen bonds between each other. If we put some non-polar molecules such as a hydrocarbons (oil) the water molecules have a harder time of orienting and have less translational and rotational entropy. The second law of thermodynamics states that processes tend to the least free energy (Gibbs energy). So the water and hydrocarbons will repell each other and eventually separate.
Importance of studying non-covalent bonds
Although large molecules are held together by covalent bonds, their shape depend much less on covalent bonds and much more by hydrogen, ionic and van der waals bonds. They determine the shape in which long molecules fold.
Understanding Properties of Molecules
Membrane
The membrane of a cell is composed of a hydrophobic molcule called a hydrocarbon.
Adding an OH (a hydroxyl group) to the hydrocarbon makes it into an alcohol, so it can dissolve in water. It becomes hydrophillic.
You could also make a hydrocarbon hydrophillic by adding a carboxyl group
Hydroxyl (OH) and carbonyl (C=O). Carbon chains with a carboxyl group are called fatty acids.
A chemical reaction happens when glycerol reacts with three different fatty acids. The hydroxyl groups are removed and create water and the fatty acids combine to create a triglyceride.
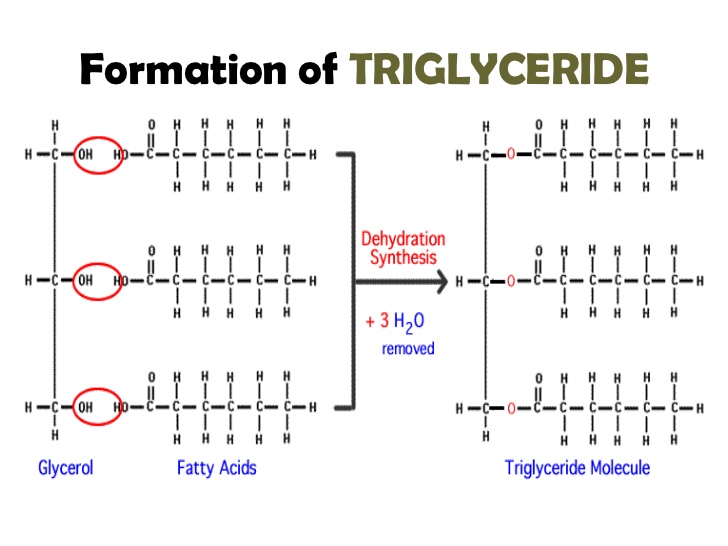
This reaction is called dehydration synthesis because a molecule is synthesized and as a by-product water is removed.
Triglyceride is a very hydrophobic molecule.
If we take two hydrocarbons and add to them a glycerol and a phosphate group which is negatively charged, we get a molecule which is amphipathic meaning it has both a hydrophilic and a hydrophobic part.

The head of the molecule is hydrophilic and the tail is hydrophobic. Imagine we have a bunch of these molecules. The system is going to tend to a low energy state. One possible local optimum is when the molecules form a membrane: the heads point out towards the water solution in the tails point inside. This formation is called a Micelle (pronounced my cell).
Another possible low energy configuration is a bilipid layer.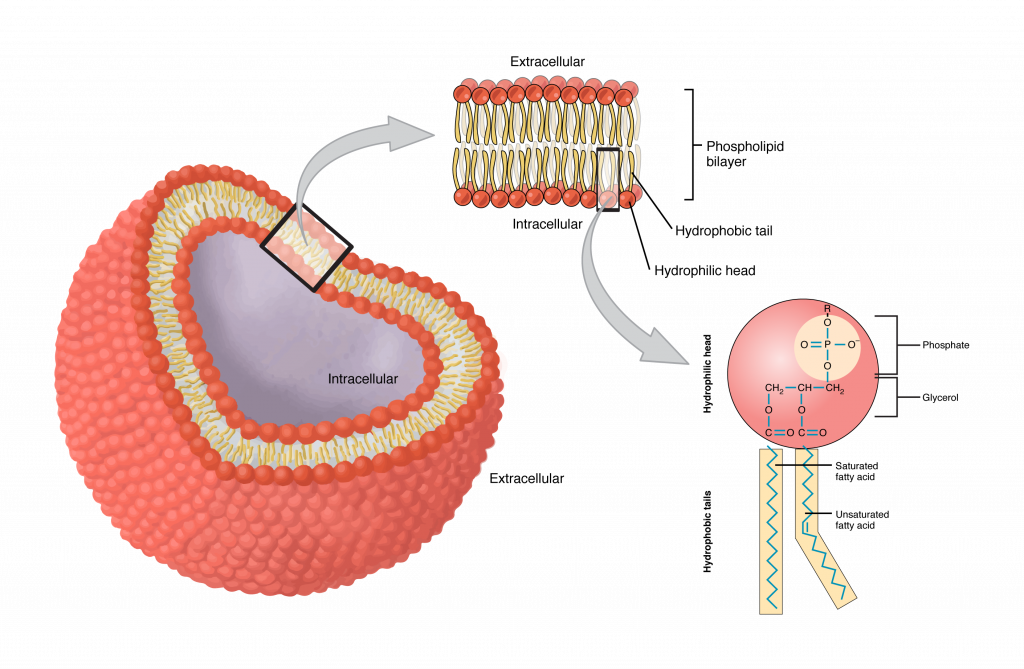
High Energy Molecule
Cells stores energy in molecules called ATP which are composed of an adenosine group with three phosphates attached to it. There has been some debate why the body stores energy with phosphate bonds.
The myth of the ‘high-energy phosphate bond’ It is frequently, and misleadingly, supposed that the phosphate anhydride bonds of ATP are ‘high-energy’ bonds which are capable of storing energy and driving reactions in otherwise unfavourable directions.
Carbohydrates
Carbohydrates usually have the following structure
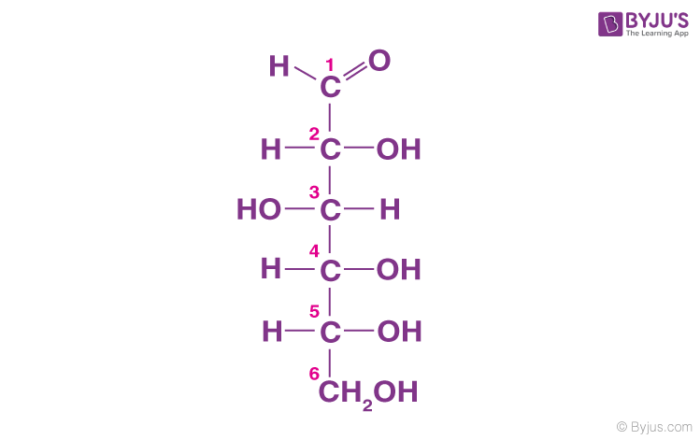
A chain of carbons with a carboxyl group at one end and a hydroxyl group at the other end. The hydroxyl group is very reactive. It makes the molecule non-polar and hydrophilic.
Though glucose usually does not appear as it does in the figure above it usually looks like this
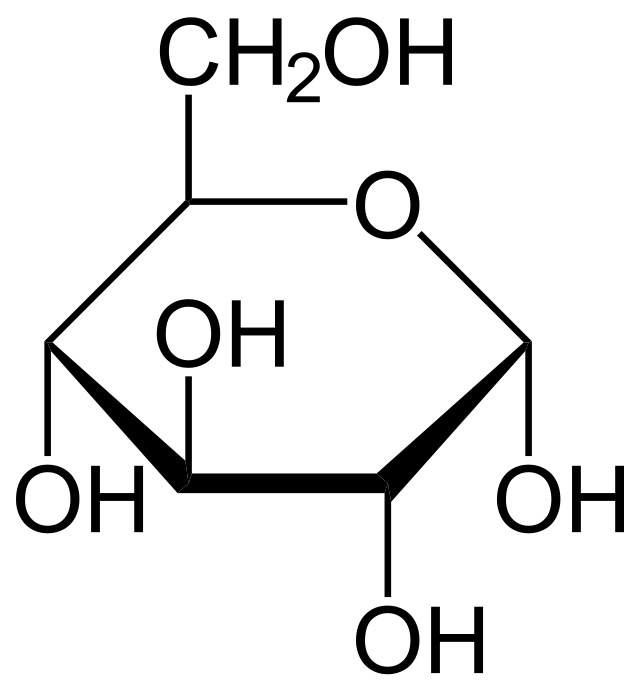
Monosacharides (single sugar molecules) can form together to make a disaccharide. Or even more sugars can form even longer molecules such as starch
 or cellulose molecules or glycogens.
or cellulose molecules or glycogens.
Proteins
Primary Structure
The building blocks of proteins are called amino acid. There are composed of a carbon called the “alpha carbon” and it has 4 groups: hydrogen, amine group, carboxyl and a sidechain.
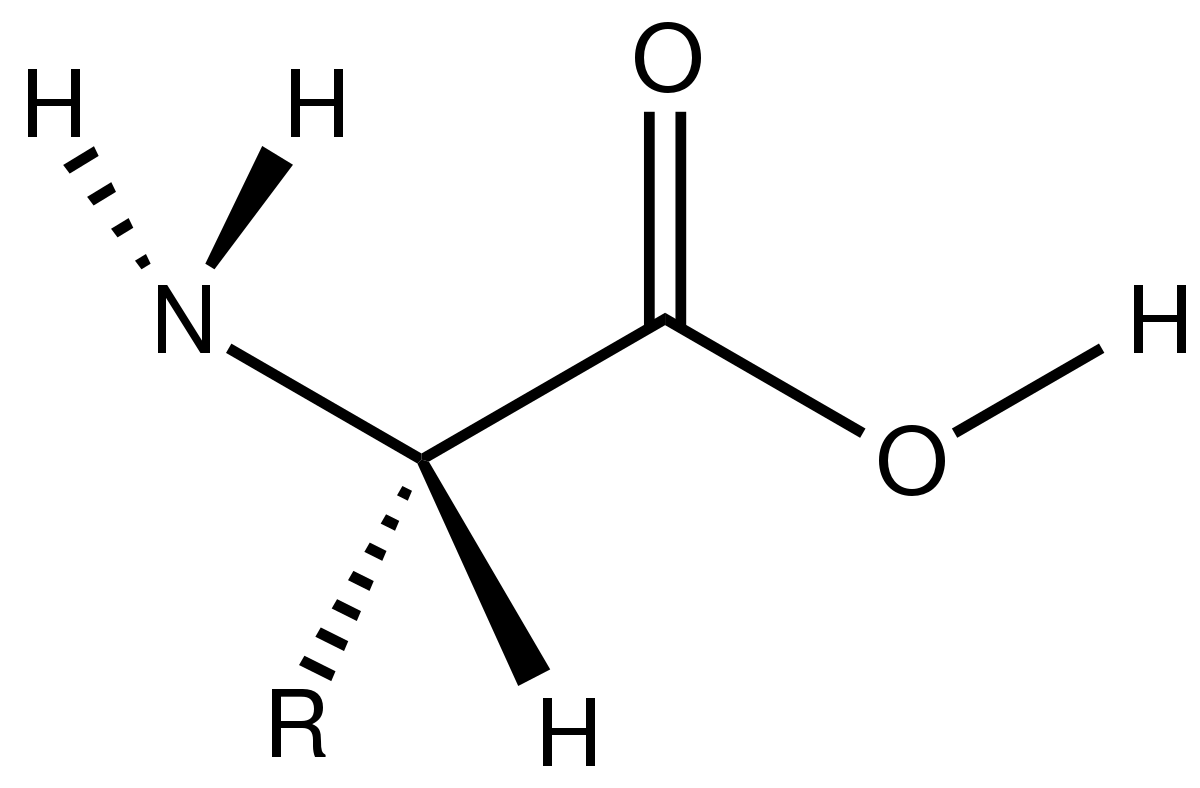
In this image, the $R$ stands for the sidechain. It can be one of 20 molecules.
Protein form chains by a reaction we already saw called dehydration synthesis.
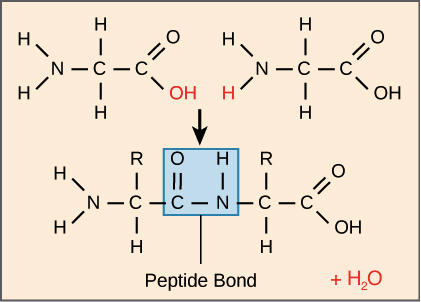 The carboxyl group and the amine group form to create a peptide bond, and a water molecule falls off.
The carboxyl group and the amine group form to create a peptide bond, and a water molecule falls off.
We could keep going to connect more and more amino acids.
each amino acid can rotate around the plane formed between the two alpha carbons.

Categories of amino acids
Polar; Uncharged
- Serine (Ser) - $CH_{2}OH$ is polar because it has $OH$ group
- Threonine (Thr)
- Asparagine (Asn)
- Glutamine (Gln)
Polar; Charged (-)
- Aspartic Acid (Asp)
- Glutamic Acid (Glu) - has an extra carbon compared to Aspartic Acid
Polar; Charged (+)
- Lysine (Lys)
- Arginine (Arg)
- Histidine (His)
Hydrophobic
- Alanine (Ala)
- Valine (Val)
- Methionine (Met) - has a sulfur
- Leucine (Leu)
- Isoleucine (Ile) - isomer of leucine
- Phenylalanine (Phe)
- Tyrosine (Tyr)
Special
- Glycine (Gly) - only H
- Proline (Pro) - hydrophobic chain bonded to nitrogen (technically it is an imino acid)
- Cysteine (Cys) - has sulfur atom - two Cysteines can bond when they come next to each other (disulfur bond)
Secondary structure
Linus Pauling came up with a classification of protein structure.
His key insight was to simplify and ignore the effect of side-chains on the structure of proteins
- Alpha-helix
- Beta-sheets
- The rest.
Tertiary & Quaternary strucure.
Tertiary structure: the mix of motifs (secondary structures) in the protein.
Quartenary structure: proteins can bond to each other. For example: two cysteines can make a covalent bond.
